IAN Research Findings: Special Diets
Date First Published: November 11, 2008
Discuss this article
The Interactive Autism Network (IAN), the nation's largest online autism research project, reports that more than 16% of families participating in the project are using special diets to treat their child with autism. What research supports the use of these diets? What symptoms do they target? What do families report about their experiences with the diets so far?
Please Note: These Findings Are Preliminary
The analyses presented here by the Interactive Autism Network are preliminary.
They are based on information submitted over the Internet by parents of children
with autism spectrum disorders (ASDs) from the United States who choose to participate.
The findings may not generalize to the larger population of children with ASDs.
The data have not been peer-reviewed -- that is, undergone evaluation by researchers
expert in a particular field -- or been submitted for publication. IAN views
participating families as research partners, and shares such preliminary information
to thank them and demonstrate the importance of their ongoing involvement.
We encourage autism researchers investigating these topics to apply for access to the IAN database. Contact researchteam@ianproject.org.
Diets and Behavior
Diet is one major way to affect the human body. It is no wonder, then, that diets have been used for many health reasons: to slim down, to control blood sugar, or to lower cholesterol. Diet also has been directed at modifying behavior, especially children's behavior, since at least the 1920s. 1
One popular target of dietary intervention has been attention-deficit hyperactivity disorder (ADHD). The Feingold diet, which eliminates food additives, such as artificial flavors and colors, as well as naturally occurring salicylates -- aspirin-like chemicals found in many fruits and vegetables -- has been particularly popular. So have diets that eliminate sugar. 2
Special diets also have long been used to treat symptoms of autism, and their use is fairly widespread. 3,4 Researchers who have surveyed families of children with ASD report frequent use of these diets, with figures ranging from 15% to 38% depending on the study and whether a family is asked if they are currently using a diet or they have ever used one. 5,6,7,8
Most of the diets being used to treat autism involve removing one or more specific substances from a child's diet, whether that substance is wheat (or gluten), milk (or casein), soy, sugar, yeast, eggs, or artificial colors, flavors, preservatives, or sweeteners. Whatever the case, it is believed that a certain substance is either causing autism symptoms or making them worse. Its removal should therefore help matters.
In some cases, a child has been tested for food allergies or sensitivities, and the diet is simply a response to the results by removing the offending item from the menu. In others, the diet is based on more far-reaching theories regarding what causes autism and attempts, within the framework of a specific theory, to intervene.
The autism-focused diets in most common use are the gluten free, casein free, or gluten-free/casein-free (GFCF) diets. Gluten is a substance found in wheat, rye, and barley, as well as products containing these. Casein is found in milk and other dairy products.
Allergies, Immune Response, and a "Leaky Gut"
Why are wheat and milk at the heart of the most popular dietary interventions in autism? In brief, there is a hypothesis that, in certain individuals with ASDs, substances resulting from the breakdown of gluten and casein are passing through the intestines to reach the bloodstream and the rest of the body. Once there, it is proposed, they create major problems.
There are several variations of this idea. All begin with the notion that some people with ASDs have intestines that are more permeable, or "leaky," than they ought to be. The cells lining the intestines are supposed to keep many potentially toxic substances out of the bloodstream, away from the rest of the body and especially the brain. A "leaky gut" lets these through. Once they are through, they can have a variety of negative effects. 9,10
Through the digestive process, casein and gluten break down into peptides. The original "leaky gut" hypothesis emphasized that these peptides are opioids -- substances that behave much like morphine in the body, impacting the development and functioning of the brain. 11,12 It also has been suggested that children with ASDs have an allergic reaction to such peptides. 13 A more recent view is that these peptides may evoke a dysfunctional nonallergic immune response. Activation of mast cells, which play a major role in allergic reactions, immunity, and inflammation, may lead to a cascade of events resulting in gut-blood-brain barrier permeability with ASD and gastrointestinal (GI) problems as the end result. 14,15
One group of researchers investigated whether it is only children with ASD and GI abnormalities who have some kind of immune reaction to common dietary proteins like those in milk. They compared immune response in children with ASD who had GI problems (some on restricted diets and some not); children with ASD who did not have any GI problems (some on restricted diets and some not); children with nonallergic food hypersensitivity (some on restricted diets and some not); and typical children. Their findings suggest there may be defects of innate immune response in children with ASD and GI issues but not in children with ASD and no GI issues. 16
As we consider discussions of diet and autism, it is interesting to note that
there is a parallel debate surrounding another disorder. In the late 1960s,
a link between celiac disease -- which involves a severe sensitivity to gluten
-- and schizophrenia was proposed. 17,18 Today, the
hypothesized connection between gluten sensitivity and this very serious mental
illness is still being explored. Many of the same issues, such as gut permeability
and toxic substances that may reach the brain to induce psychosis, have been
raised. 19,20,21,22
The GFCF Diet -- So Far, Limited Research Findings
Diets are not easy to study. How do you enforce the restrictions of a diet, making sure those who are supposed to be on it really are? How do you keep participants, their parents, and researchers "blinded" so that their hopes, fears, expectations, and biases do not warp observations or results? How do you untangle other factors -- like the fact a parent may pay more attention to a child or a family may have a more orderly routine when the diet is underway and researchers are keeping track -- from the effects of the diet itself?
Because of the difficulties, there are few studies on the GFCF diet that approach the gold standard of research: the randomized controlled trial (RCT). Such a study involves:
(To learn more about RCTs, read Gold Standard of Evidence: The Randomized Controlled Trial [RCT].)
So far, there have been only two randomized controlled trials conducted on the GFCF diet, with mixed results. 23 In Norway, Knivsberg and colleagues worked with children who had a diagnosis of autism as well as peptides in their urine -- a sign of "leaky gut" as described earlier. Only 20 children participated in this 12-month study, 10 on the diet and 10 in the control group. The people judging the outcome were "blinded;" they didn't know who had been on the diet and who had not. Children, parents, and researchers all knew who had been on the diet, however. Children who received the intervention did improve compared with those who had not. Their autistic trait and social interaction scores were lower, while overall ability to communicate and interact improved.
Another study, this one taking place in the United States, recruited 13 children with autism. The researchers placed half the group on the GFCF diet and half on a regular diet for six weeks, then switched the groups and continued for another six weeks. In this study, parents and observers were "blinded." 24 In contrast to the Norwegian study, no significant effects of the diet were found. In addition, eight parents were unable to correctly identify when their child had been on or off the diet. Despite this, parents of seven of the children reported improvements in language, decreased hyperactivity, and decreased tantrums.
Were the researchers missing something, or were hopeful parents seeing something that wasn't there? It has been shown that parental expectations can influence how a child's behavior and progress are viewed. In one study, for example, parents who were told their child had received a sugar drink rated their child as more hyperactive than did parents who were told their child received a sugar-free drink. (In truth, all the children in this study were given a sugar-free drink.) 25
Consider the case of secretin. Following a news story reporting that a child with autism dramatically improved after receiving this hormone during a medical procedure, secretin was hailed as a possible cure for autism. A number of clinical trials later showed that secretin was not effective in the treatment of autism. 26 In one such study, 30% of both the group receiving secretin and the group receiving a saline-solution placebo showed improvement, according to parent and teacher reports. The researchers concluded that secretin was not an effective treatment for ASD compared with the placebo. Regardless, 75% of participating parents, when informed of the study's result, continued to express belief in the potential benefit of secretin as an autism treatment. 27
This demonstrates why having a control group and "blinded" observers is so important. Based on parent and teacher report alone, and without a placebo-controlled group for purposes of comparison, secretin would have appeared to be effective in at least some cases.
Now it is the GFCF diet that needs to be put to the test. The two trials done so far were contradictory and extremely small. Considering the widespread use of the GFCF diet, much larger randomized controlled trials are urgently needed. Fortunately, at least one of these is now being carried out by the National Institute of Mental Health.
One crucial issue will be whether the diets are effective (or not) for specific groups of children with ASD. Are there certain subgroups, such as those with GI issues, who are most likely to be helped by this intervention? Are there other groups for whom the diets do little or nothing? To guide treatment decisions, it is crucial to identify not only whether a treatment is effective, but also for which group of children.
IAN Families Report on Diets
What have families participating in the IAN Project reported about special diets?
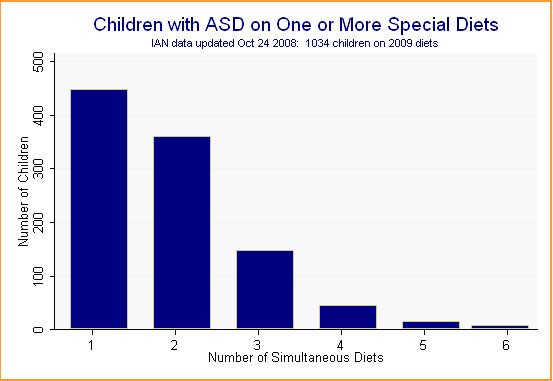
IAN families have reported on 32,820 separate treatments. Of these, 2,009 -- or 6.1% -- are special diets. In many cases, children are on more than one diet at a time. Of the 6,295 children whose parents have provided treatment data, 1,034 -- or 16.4% -- are on at least one special diet. (See Figure 1.)
Figure 1.

Much of the overlap in diet use is the result of thorough parents reporting on the gluten-free, casein-free, and gluten-free/casein-free (GFCF) diets. Even accounting for such duplication, which reduces our total diets from 2,009 to 1,318, the GFCF diet is by far the most frequently used dietary intervention. Diets focused on removal of artificial colors, flavors, preservatives, and/or sweeteners are a distant second, followed by sugar- or carbohydrate-free or -restricted diets, yeast-free diets, soy-free diets, and a variety of others. (See Figure 2.)
Figure 2.
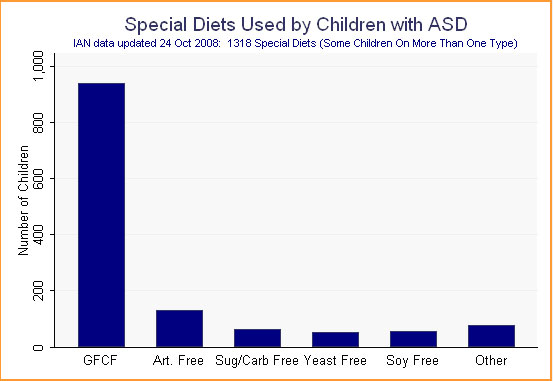
Because the GFCF diet is clearly the number-one dietary intervention being used by families, we will focus the remainder of our analysis on this diet. We have placed in this category all gluten-free, casein-free, gluten-free/casein-free, or lactose/dairy-free diets.
How Did Families Learn About the GFCF Diet?
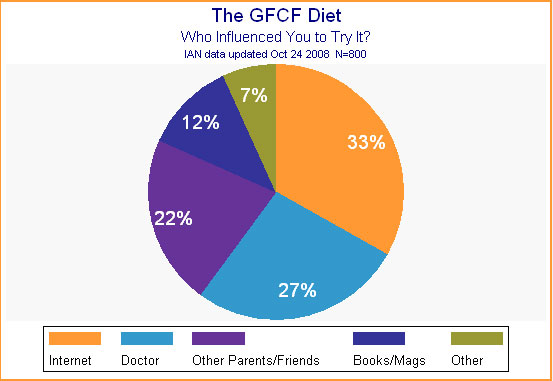
Because the GFCF diet is considered a "complementary and alternative" treatment by many, we wondered how families had learned about it. Who had most influenced them to try it? Despite its possible status as an alternative treatment, 27% of the 800 families who answered this question were influenced by their doctor to try it. Thirty-three percent were convinced to try it by what they had read on the Internet, while another 22% said other parents or friends had recommended this intervention. (See Figure 3; percentages add up to more than 100% due to rounding.)
Figure 3.
Targeted Symptoms
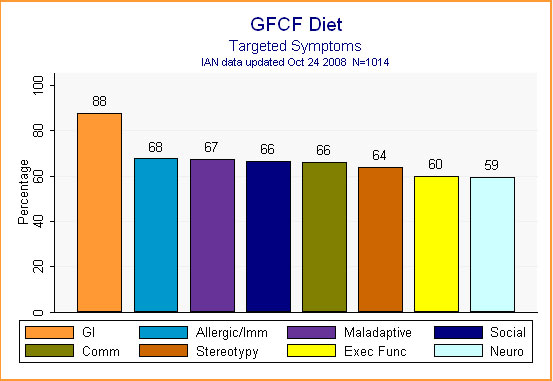
What symptoms did families hope the GFCF diet would alleviate? Looking at Figure 4, it is clear that it was hoped the diet would treat a wide variety of symptoms, from social relatedness to executive function. The most frequent symptom of concern, however, was gastrointestinal distress -- which makes sense considering the theories underpinning the diet.
Figure 4.
Costs, Barriers, and Benefits
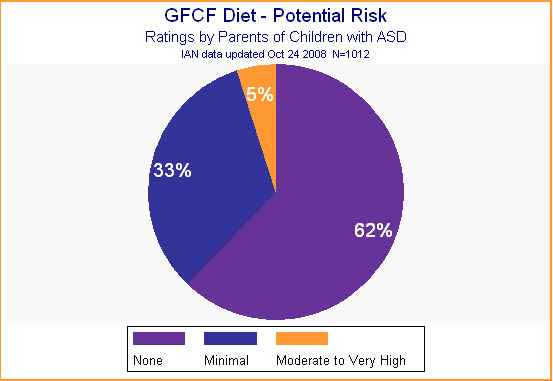
Parents wrestle with many pros and cons when making treatment decisions. These include weighing the cost of the treatment, the risk it involves, and other burdens it will impose against hoped-for results. Of course, believing a treatment at least will do no harm is an attractive point in a treatment's favor. One likely reason for the popularity of the GFCF diet is that 95% of parents felt that the diet presented no or minimal risk. (See Figure 5.)
Figure 5.
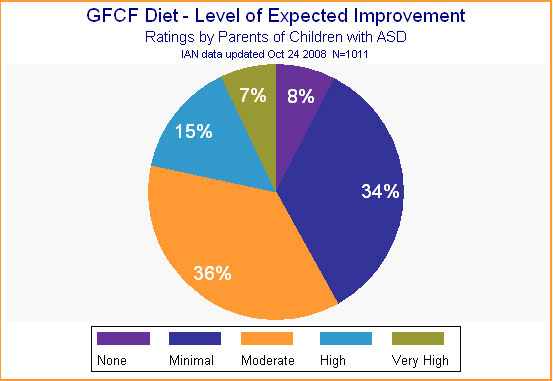
Expectations for improvement, on the other hand, were quite high. More than half the parents expected the GFCF diet to yield at least moderate improvement in their child. (See Figure 6.)
Figure 6.
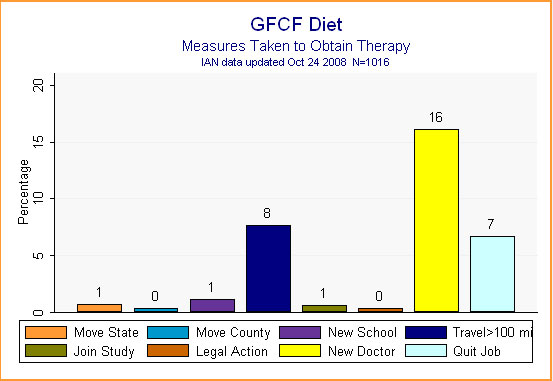
There were some extraordinary and inconvenient measures parents didn't need to take to obtain the GFCF diet. Only 16% said they had needed to find a new doctor to oversee the diet, while 8% said they had had to travel more than 100 miles (perhaps to see this doctor or to find specialized food products). Seven percent said they had had to quit a job, possibly to oversee and manage their child's special diet. (See Figure 7.)
Figure 7.
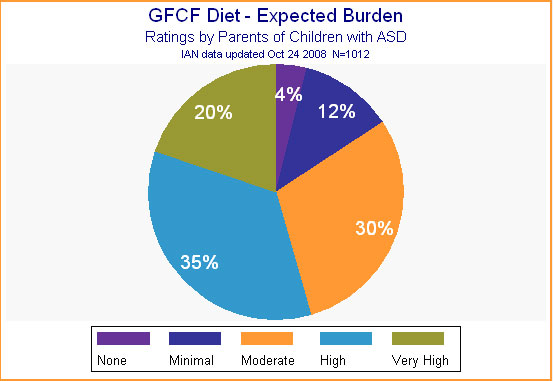
Parents didn't expect the diet to be free of inconvenience, however. As shown in Figure 8, more than 80% expected a moderate to very high level of burden when starting this intervention. After all, a diet may involve making menus, learning new recipes, changing family habits, and coping with a child's resistance to a new routine or to new foods. In addition, removing all wheat and milk eliminates many everyday foodstuffs, from crackers to cheese, from bread to ice cream, not to mention most fast food. The GFCF diet therefore requires shoppers to read every label, and to find substitutes for many common foods. These substitutes may be expensive, available only at specialized stores or by mail order.
Figure 8.
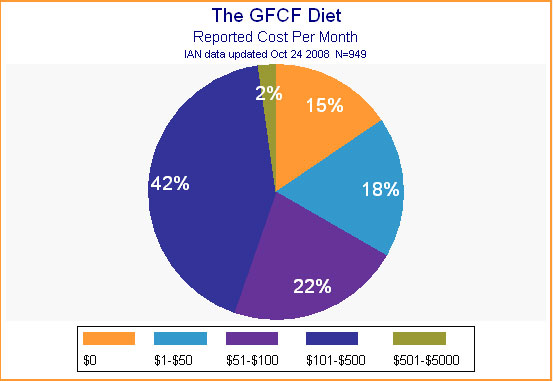
It may be that parents were willing to take on the expected burden in exchange for what they perceived as low risk combined with high potential for improvement. As for financial impact of the diet, the majority of parents reported that it cost somewhere between $50 and $500 per month to provide it. (See Figure 9.)
Figure 9.
Diet Results
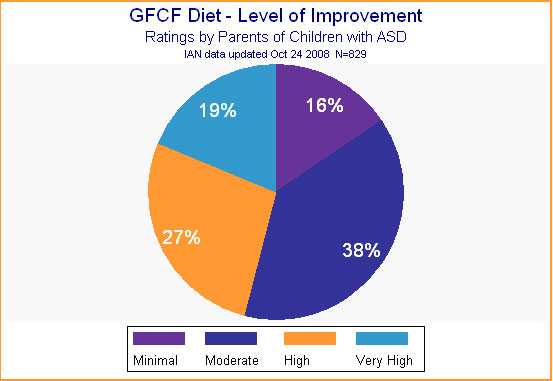
After a child begins a diet, most parents are pleased with the results. The vast majority (82%) report definite improvement in their child's skills. How much improvement? That varied a great deal, as shown in Figure 10. About half reported a "minimal" or "moderate" level of improvement, while the other half reported a "high" or "very high" level of improvement.
Figure 10.
A word of caution: The information reported here reflects only parents' evaluation of current treatments. Because any treatment a child is currently receiving must be considered worthwhile by parents, we expect ratings to be fairly high at this point. As data are collected over time, and families can report on treatments they have dropped, there likely will be more negative reports, not just on this treatment, but on many treatments. Add to this the major impact of the placebo effect found in many autism treatment studies, and it is wise to take these findings as very preliminary and as yet incomplete.
If You Try the Diet -- Things to Keep in Mind
If you are considering trying the GFCF diet, there is one important step you can take to keep risk as low as possible. A major concern is making sure growing children receive sufficient nutrition as certain aspects of their diet become restricted. For example, one study showed that children with ASD had thinner finger bones than other children, and this was especially true for those on casein-free diets. 28 Make sure to consult with your pediatrician or another physician or nutritionist if you decide to undertake any kind of special diet.
REFERENCES
1. Cormier, E., & Elder, J. H. (2007). Diet and child behavior problems:
Fact or fiction? Pediatric Nursing, 33(2), 138-143.
2. Weber, W., & Newmark, S. (2007). Complementary and alternative medical
therapies for attention-deficit/hyperactivity disorder and autism. Pediatric
Clinics of North America, 54(6), 983-1006; xii. t
3. Levy, S. E., Mandell, D. S., Merhar, S., Ittenbach, R. F., & Pinto-Martin,
J. A. (2003). Use of complementary and alternative medicine among children recently
diagnosed with autistic spectrum disorder. Journal of Developmental and Behavioral
Pediatrics: JDBP, 24(6), 418-423. View Abstract
4. Wong, H. H., & Smith, R. G. (2006). Patterns of complementary and alternative
medical therapy use in children diagnosed with autism spectrum disorders. Journal
of Autism and Developmental Disorders, 36(7), 901-909.
5. Green, V. A., Pituch, K. A., Itchon, J., Choi, A., O'Reilly, M., & Sigafoos,
J. (2006). Internet survey of treatments used by parents of children with autism.
Research in Developmental Disabilities, 27(1), 70-84.
6. Harrington, J. W., Rosen, L., Garnecho, A., & Patrick, P. A. (2006).
Parental perceptions and use of complementary and alternative medicine practices
for children with autistic spectrum disorders in private practice. Journal of
Developmental and Behavioral Pediatrics: JDBP, 27(2 Suppl), S156-61.
7. Witwer, A., & Lecavalier, L. (2005). Treatment incidence and patterns
in children and adolescents with autism spectrum disorders. Journal of Child
and Adolescent Psychopharmacology, 15(4), 671-681.
8. Hanson, E., Kalish, L. A., Bunce, E., Curtis, C., McDaniel, S., Ware, J.,
et al. (2007). Use of complementary and alternative medicine among children
diagnosed with autism spectrum disorder. Journal of Autism and Developmental
Disorders, 37(4), 628-636. View Abstract
9. D'Eufemia, P., Celli, M., Finocchiaro, R., Pacifico, L., Viozzi, L., Zaccagnini,
M., et al. (1996). Abnormal intestinal permeability in children with autism.
Acta Paediatrica (Oslo, Norway: 1992), 85(9), 1076-1079.
10. Liu, Z., Li, N., & Neu, J. (2005). Tight junctions, leaky intestines,
and pediatric diseases. Acta Paediatrica (Oslo, Norway: 1992), 94(4), 386-393.
11. Knivsberg, A. M., Reichelt, K. L., Hoien, T., & Nodland, M. (2002).
A randomised, controlled study of dietary intervention in autistic syndromes.
Nutritional Neuroscience, 5(4), 251-261. View Abstract
12. Reichelt, K. L., & Knivsberg, A. M. (2003). Can the pathophysiology
of autism be explained by the nature of the discovered urine peptides? Nutritional
Neuroscience, 6(1), 19-28.
13. Lucarelli, S., Frediani, T., Zingoni, A. M., Ferruzzi, F., Giardini, O.,
Quintieri, F., et al. (1995). Food allergy and infantile autism. Panminerva
Medica, 37(3), 137-141.
14. Jyonouchi, H., Geng, L., Ruby, A., Reddy, C., & Zimmerman-Bier, B. (2005).
Evaluation of an association between gastrointestinal symptoms and cytokine
production against common dietary proteins in children with autism spectrum
disorders. The Journal of Pediatrics, 146(5), 605-610.
15. Theoharides, T. C., Doyle, R., Francis, K., Conti, P., & Kalogeromitros,
D. (2008). Novel therapeutic targets for autism. Trends in Pharmacological Sciences,
29(8), 375-382.
16. Jyonouchi, H., Geng, L., Ruby, A., & Zimmerman-Bier, B. (2005). Dysregulated
innate immune responses in young children with autism spectrum disorders: Their
relationship to gastrointestinal symptoms and dietary intervention. Neuropsychobiology,
51(2), 77-85.
17. Dohan, F. C. (1966). Wheat "consumption" and hospital admissions
for schizophrenia during World War II. A preliminary report. The American Journal
of Clinical Nutrition, 18(1), 7-10.
18. Dohan, F. C. (1969). Is celiac disease a clue to the pathogenesis of schizophrenia?
Mental Hygiene, 53(4), 525-529.
19. Bushara, K. O. (2005). Neurologic presentation of celiac disease. Gastroenterology,
128(4 Suppl 1), S92-7.
20. Kalaydjian, A. E., Eaton, W., Cascella, N., & Fasano, A. (2006). The
gluten connection: The association between schizophrenia and celiac disease.
Acta Psychiatrica Scandinavica, 113(2), 82-90.
21. Peleg, R., Ben-Zion, Z. I., Peleg, A., Gheber, L., Kotler, M., Weizman,
Z., et al. (2004). "Bread madness" revisited: Screening for specific
celiac antibodies among schizophrenia patients. European Psychiatry: The Journal
of the Association of European Psychiatrists, 19(5), 311-314.
22. Wei, J., & Hemmings, G. P. (2005). Gene, gut and schizophrenia: The
meeting point for the gene-environment interaction in developing schizophrenia.
Medical Hypotheses, 64(3), 547-552.
23. Millward, C., Ferriter, M., Calver, S., & Connell-Jones, G. (2008).
Gluten- and casein-free diets for autistic spectrum disorder. Cochrane Database
of Systematic Reviews (Online), (2)(2), CD003498.
24. Elder, J. H., Shankar, M., Shuster, J., Theriaque, D., Burns, S., &
Sherrill, L. (2006). The gluten-free, casein-free diet in autism: Results of
a preliminary double blind clinical trial. Journal of Autism and Developmental
Disorders, 36(3), 413-420.
25. Hoover, D. W., & Milich, R. (1994). Effects of sugar ingestion expectancies
on mother-child interactions. Journal of Abnormal Child Psychology, 22(4), 501-515.
26. Williams, K. W., Wray, J. J., & Wheeler, D. M. (2005). Intravenous secretin
for autism spectrum disorder. Cochrane Database of Systematic Reviews (Online),
(3)(3), CD003495.
27. Sandler, A. (2005). Placebo effects in developmental disabilities: Implications
for research and practice. Mental Retardation and Developmental Disabilities
Research Reviews, 11(2), 164-170.
28. Hediger, M. L., England, L. J., Molloy, C. A., Yu, K. F., Manning-Courtney,
P., & Mills, J. L. (2008). Reduced bone cortical thickness in boys with
autism or autism spectrum disorder. Journal of Autism and Developmental Disorders,
38(5), 848-856.